Mounting evidence from basic research over the past decade reveals that inflammation and endothelial dysfunction are the central processes in all stages of atherosclerosis; determining levels of the inflammatory mediators (cells such macrophages, or enzymes such as matrix metalloproteinase) accumulated in the plaque would be a way to estimate vulnerability. We have modified human low density lipoprotein (isolated from donor blood) and turned them into nanoparticles carrying a GdDPTA contrast agent. MRI can visualize their uptake by the macrophages in the atheroplaque of apoE-/- mice.
We also developed high-density lipoprotein (HDL)-like nanoparticle that contains iron oxide clusters whose uptake in the atheroplaque is confirmed by Prussian blue staining.
Figure 1.
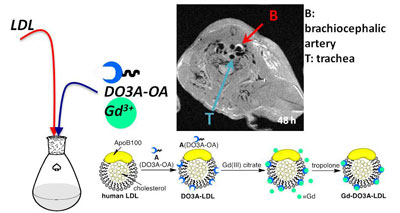
Figure 2.
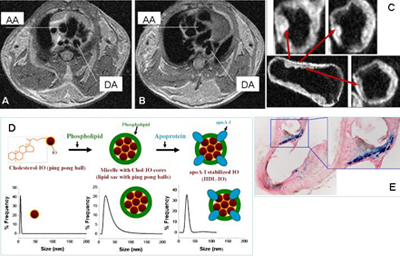
Arteriolosclerosis in LDLr-/- mouse detected by in vivo (A-B) and ex vivo (C) MR imaging. Diagram of lipoprotein-iron oxide (IO) nanoparticles (D) and Prussian blue staining of artery segments after injection of nanoparticles (E). AA= ascending aorta, DA= descending aorta.
Our laboratory is focused on two aspects of stem cell mediated cardiac repair:
- Develop in vivo imaging methods to track the fate (survival, proliferation and differentiation) of stem cells and evaluate their effects on global and regional cardiac function. We have developed magnetic resonance imaging (MRI) methods to measure the global and contractile function in living mice.
- Examine embryonic stem cell (ESC) derived cardiomyocytes (CM) for the treatment of an infarcted heart. Utilizing murine ESCs, carrying an early cardiac specific gene whose expression is linked to an antibiotic resistant gene, we are able to purify the CM population from beating embryoid bodies and obtain sufficient number of cells for in vivo study.
The long-term goal of this project is to facilitate the clinical translation of stem cell mediated therapy for myocardial infarction by identifying the optimal cell type (ESCs, skeletal myoblasts, bone marrow derived or resident cardiac progenitor cells) and understanding the mechanism of their capability to improve the cardiac function.
Figure 1.

The long (LA) and short (SA) axis view of a rat heart receiving 5 million ESCs, that are stably transfected with a reporter gene (HSV1-sr39tk) and labeled with SPIO particles. [F-18]FHBG signal from grafted cells are in color while myocardial wall is delineated by [N-13] ammonia signal in grey scale. Hypointense region on MR images early after injection represent distribution of cells in the myocardium (a). Over time, the hypointense area reduces while contrast-to-noise ratio remains (b) reflecting the fact that SPIO labels are diluted due to proliferation of ESCs and labels in the dead cells are transferred to macrophages as demonstrated by double staining of SPIO (c) and CD68 (d). The overlay of the two suggests that the majority of SPIO positive cells are infiltrated macrophages.
Figure 2.
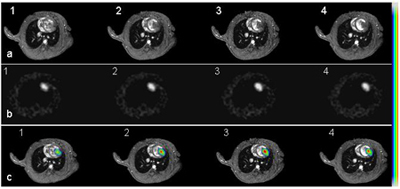
Short axis cardiac MR images of an infarcted heart 14 days after receiving 5M double-labeled ESCs (a, panel 1-4 from base to the apex of the heart). b: [F-18]FHBG images acquired from the same animal. These images have been reformatted to the short axis views as MR images. c: Co-registered PET and MR images using a mutual-information based algorithm. Color scale was used for PET signal while grey scale for MR images.
- Under an approved IRB, explanted hearts are procured from the UPenn Heart Transplantation Program. A myocardial wedge (approx. 2 gram) is dissected from the LV anterior-septal wall and cannulated at ~ 7th branch of the left anterior descending coronary artery (Figure 1). The wedge is perfused with Krebs-Henseleit buffer containing carbon-13 labeled or unlabeled substrates such as glucose, ketone bodies and palmitate. The perfusion media is oxygenated with 95/5 percent O2/CO2 and the pH is maintained at 7.4 ±0.2. The catheterized wedge is secured in a 20 mm NMR tube and then placed inside a 400 MHz NMR spectrometer. High resolution NMR is also performed on tissue extracts.
- Bonded cumulative isotopomers (Bonded Cumomers) mathematical model (Shestov et al. Neurochem Res 11: 2388-401, 2012) is used to derive the quantitative flux measurement by taking advantage of fine-structures carbon-13 MR spectra (singlet, doublet, triplet etc.) and mass balance and isotopomer balance at each (rate-limiting) step of the pathway.
Figure 1.
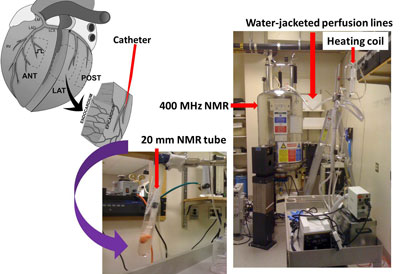
Perfused heart wedges for Carbon-13 NMR-based metabolic studies.
Non-invasive measurement of receptor and gene expression on a low density lipoprotein receptor (LDLr) knockout mouse model, we have developed MR imaging and spec method to evaluate the restoration of LDLR gene expression.
The long term goal of this project is to develop a report-gene approach combined with non-invasive imaging/spectroscopic methods for quantification of transgene expression in the liver, which is a major target organ for gene therapies aimed at correcting metabolic disorder (such as familial hypercholesterolemia) or cancer.
Figure 1.
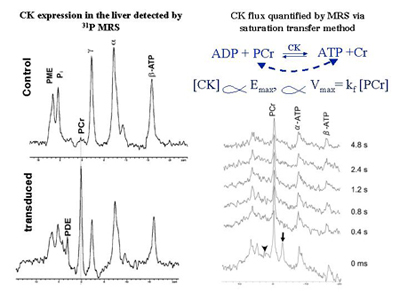
Creatine kinase (CK) expression in the mouse liver detected by in vivo 31P MR spectroscopy (left panel) and quantification of CK concentration (proportional to CK flux) by a progressive saturation transfer experiment (right panel). The phosphocreatine (PCr) resonance only occurs in the liver transfected with creatine kinase (CK), which is not normally expressed in the liver. The ɣ-ATP (arrow head) is saturated for the time indicated on the right side.